
Medtec Grand Prix KOBE2021
Standing up for solutions to society's problemsDrug discovery, medical devices, regenerative medicine, healthcare, research tools, etc.
We implement the technologies and thoughts of entrepreneurs and researchers in the MedTech area into society together.
News

- Grand Prix
- News
- Medtec Grand Prix KOBE

- News
- Medtec Grand Prix KOBE

- News
- Medtec Grand Prix KOBE

- Medtec Grand Prix KOBE
Outline of the event
- point (e.g. of a statement)
- Scientific and technological "seeds" are being created in universities, research institutes, and corporate laboratories, but it takes a lot of effort before they sprout into practical applications. The "Tech Planter," organized by RIVANES and its partners, is an ecosystem that aims to serve as a planter to discover these seeds and help them sprout into business. The "MedTech Grand Prix KOBE2021" is a program aimed at discovering and fostering technology seeds and entrepreneurs in the real tech field (drug discovery, medical devices, regenerative medicine, healthcare, research tools, etc.).
Related Grand Prix :. Medtec Grand Prix KOBE2023 - subject (of taxation, etc.)
-
- Those who have a vision to change the world or improve the world based on the technological seeds in the real tech field and have a will to establish or develop a new business.
- Individuals and teams are both welcome to participate, even before incorporation.
- No limitation on the number of years of establishment, even if you are already a corporation. Even if you are close to mass production or PMF establishment, if you are planning to collaborate with partner companies, etc., it is acceptable.
- Application Theme
- New prevention, diagnosis and treatment
- Application period
- Monday, March 1, 2021 - Friday, July 9, 2021
- Screening Criteria
-
A panel of judges composed of LIVERNESS and its partners will review the following items
- 01novelty (patentability)
- 02feasibility
- 03Are you going to change the world?
- 04passion
- commendation
- Grand Prize: (300,000 yen cash prize + right to receive 5 million yen business investment) 1
Corporate Prize: (200,000 yen) Approximately 10 prizes will be awarded.
Schedule
- 3/1[MON]
-
Entry Start
this way (direction close to the speaker or towards the speaker)Please register as a member and fill out the web form to apply.
We also hold information sessions on a college-by-college and individual basis to encourage determination to enter and to teach tips on document preparation. Please feel free to contact us for more information.
- 6/26[SAT]
-
kickoff
RIVANES and its partners, as well as entered teams, will meet and mingle.
Business consultation and partnership development with each other will be available. Participation or lack of participation on the day of the event will not be considered.
- 7/12[MON]
-
1st round: Application Screening
A panel of judges composed of LIVERNESS and its partners will review the documents.
You will be asked to submit information about your team, skills, passions, and vision for the future via web form and video.
All applicants will be notified of the screening results and provided with feedback on their applications.
In addition, individual interviews (also online) for presentation review will be conducted for those who wish to participate.
- 8monthfirst 10 days of month
-
Finalists determined
Interviews will be held for applicants who pass the document screening to brush up their presentations for the final round.
In addition, meetings can be set up with partner companies for the purpose of business consultation and developing partnerships.
- 10/9[SAT]
-
Medtec Grand Prix KOBE
The 12 finalist teams that pass the document screening will be screened for presentation.
- 10Month~
-
Matching Support Period
After the Demo Day, the contact point between the venture and LIBANES will continue to be open for discussions on commercialization.
We handle a wide range of start-up issues, from incorporation to prototype development, financing, R&D, and more.
In addition, collaborative matching between ventures and partner companies has become more active to support business development.
Partners
-

Kawasaki Heavy Industries, Ltd. -

Sysmex Corporation -

Senju Pharmaceutical Co. -

SOMPO Holdings, Inc. -

Taisho Pharmaceutical Co. -

Daiichi Sankyo Company, Limited -

Dai-ichi Life Holdings Co. -

Meiji Holdings Co. -

Rohto Pharmaceutical Co.
asset
- ASSET01Riverness Communicator
- ASSET02Partners
- ASSET03Professional Supporter
- ASSET04superfactory group
Support
- SUPPORT01Organize the purpose and vision of starting a business
- SUPPORT02Brush up on your presentation
- SUPPORT03Providing opportunities for business company collaboration
- SUPPORT04Support for R&D and prototype development
- SUPPORT05Support for knowledge and IP strategies
- SUPPORT06finance
Grand Prix Outline
- Grand Prix Name
- Medtec Grand Prix KOBE2021
- Location
- Kobe University Center for Advanced Interdisciplinary Research, Convention Hall (7-1-48 Minatojima Minamimachi, Chuo-ku, Kobe, Hyogo 650-0047, Japan)
- Date & Time
- Saturday, October 9, 2021, 13:00-19:00
- Application period
- Monday, March 1, 2021 - Friday, July 9, 2021
- Participation Target
- Held on a closed, pre-registration basis (finalists, partner companies, professional supporters, Super Factory Group)
- organizing
- City of Kobe, Kobe Medical Industry Development Organization, RIVANES Co.
- timeline
-
- 12:30-13:00
- inauguration
- 13:00-13:30
- Greetings from the Organizer
- 13:30~16:50
- Final Selection Presentations (Presenter①~⑫)
- 16:50-17:50
- Review Time
- 17:50-19:00
- Announcement of Judging Results and Award Ceremony
judge
-

-
head judge Executive Officer, LIVERNESS Co.Hiroyuki Takahashi
- D. from Yokohama City University. D. in Science. He has been involved in the creation of opportunities for the fusion of different fields, such as the "Liberalness Research Grant," a private-sector-led research grant for researchers under 40 years old, and the establishment of the "Association of Academic Societies in Different Fields," which creates solutions to social issues and new research by accumulating and integrating diverse knowledge without being bound by the boundaries of academia and industry. He is working to create a mechanism to keep research revolving between academia, venture companies, and large corporations, with a focus on the life science field.
-
head judge
-
-
General Manager, Cluster Promotion Center, Kobe Medical Industry Development OrganizationTadaaki Hanaya
- After joining the Ministry of Health, Labour and Welfare, he was transferred to Fukuoka Prefectural Government, the National Institute for Health Care Excellence (NICE) in the U.K., the Cabinet Secretariat's Office for H1N1 Influenza Control, and the Kyoto University iPS Cell Research Institute, before assuming his current position at the Pharmaceutical Safety Division, Pharmaceuticals and Consumer Health Bureau, MHLW.
-
-

-
Professor, Graduate School of Health Innovation, Kanagawa University of Health and WelfareKuniko Masako
Advisor, Kobe Medical Industry Development Organization
- He has worked for foreign and domestic medical device companies, mainly in clinical, regulatory, insurance, and other development and government affairs. Currently, he is a professor at the Graduate School of Health Innovation, Kanagawa University of Health and Welfare, where he also serves as a member of AMED's Research and Management Council and as a member of the Issue Assessment Committee. He specializes in regulatory, product development, business development, insurance policy, and medical policy related to healthcare technology. During his tenure at the company, he served as an expert member of the Medical Devices Subcommittee of the Chuikyo Medical Association and an expert member of the Cost Effectiveness Subcommittee.
-
-

-
General Manager, Innovation Department, Planning Division, Kawasaki Heavy Industries, Ltd.Hideaki Odate
- Graduated from Chuo University, Faculty of Commerce, Department of Accounting in 1992, and joined Kawasaki Heavy Industries in the same year, where he was a core member in launching various new businesses and products, including FA/robotics, large structures, renewable energy, railway-related, rechargeable batteries, and MC&E. In his current position in the Innovation Department from May 2020, he leads the search for synergies for existing businesses and new businesses, as well as the establishment of an internal innovation culture. In his current position in the Innovation Department since May 2020, he leads the search for synergies and new business opportunities for existing businesses and the establishment of a culture of innovation within the company.
-
-

-
Director, Managing Executive Officer, Sysmex CorporationTomokazu Yoshida
- After graduating from Okayama University Graduate School of Pharmaceutical Sciences in 1995, he was engaged in cardiovascular research and drug discovery research in the fields of central nervous system diseases and oncology at national research institutes and pharmaceutical companies. In 2013, he became a director of MEDICALOID Corporation, where he was involved in the development and market introduction of medical robots, and in 2021 he became a director and managing executive officer (current position). (He also serves as Director and Executive Officer of Medicaloid Inc.
-
-

-
General Manager, Research and Development Division, General Research Laboratory, Senju Pharmaceuticals Co.Meiji Isowaki
- After joining the company in 1993, he was engaged in the formulation design of ophthalmic eye drops and research and development of ophthalmic DDS. In 2006, he received a doctorate in Information Technology from Kyushu Institute of Technology.
In 2009, he was transferred to the U.S. subsidiary Senju USA, Inc. where he was involved in basic research and clinical development of ophthalmic DDS formulations; after returning to Japan in 2016, he was engaged in project management in the Product Strategy Office and later became the head of the Drug Discovery Technology Development Office before being appointed as the General Research Laboratory Director in 2021.
-
-

-
Section Manager, Healthcare Business Development Department, SOMPO Holdings, Inc.Tsuneo Deguchi
- In 1998, he joined Teijin Limited (currently Teijin Pharma Limited), where he has 20 years of experience in a series of professional duties including research and development, clinical trials, and regulatory filings, mainly in the medical device field, as well as collaboration with promising domestic and overseas companies, including 6 years in the US, and new business planning and business development. In order to utilize his experience to energize Japan, he joined Sompo Japan in May 2018 and has been engaged in new business creation in his current position since April 2020.
-
-

-
Senior Manager, Frontier Research Center, Taisho Pharmaceutical Co.Maki Hirate
- Joined Taisho Pharmaceutical Co., Ltd. in 2002. He was assigned to the Lead Discovery Laboratory, where he was in charge of high-throughput screening. In 2019, he will join the Frontier Research Center, where he will work on sourcing activities for the development of innovative drug discovery technologies and new businesses related to advanced medicine. He is currently working at the Frontier Research Center for new business development in innovative drug discovery technologies and advanced medicine. He holds a Master's degree from the Graduate School of Pharmaceutical Sciences, University of Shizuoka, and was born in Hyogo Prefecture, Japan.
-
-

-
Executive Officer, General Manager of Research Management Division, R&D Headquarters, Daiichi Sankyo Company, LimitedWataru Takahashi
- In 1990, he joined the former Sankyo Company, Limited, where he worked in the Active Substances Research Laboratory. In 2013, he founded the New Modality Research Laboratories (NMR), a new research institute for drug discovery that focuses on drug discovery modalities other than small molecules, such as antibodies, peptides, and nucleic acid drugs. After serving as Director of the Biofoundation Research Laboratories and Director of the Modality Research Laboratories, he assumed his current position in 2020. From the former Sankyo era to Daiichi Sankyo, he has been consistently engaged in research and development of antibody drugs.
-
-

-
Executive Officer and General Manager, Innovation Promotion Unit, Dai-ichi Life Holdings, Inc.Takehiko Eguchi
- Graduated from Nagoya University, Faculty of Engineering, Department of Applied Physics. After working in investment development at Yamaichi Securities, he ran an internet startup for 9 years, invested in startups in Japan and overseas at SB investment firms, and was involved in new business development at large companies (Hitachi Zosen, Konica Minolta, SOMPO). MBA from Indian School of Business.
-
-

-
Meiji Holdings Co.Toru Kurosawa
Director, Managing Executive Officer, General Manager of Pharmaceutical Research and Development Division, Meiji Seika Pharma Co.
- D. in Agriculture from Niigata University. In 1990, he joined Meiji Seika and was assigned to research laboratories, where he was mainly engaged in non-clinical safety and pharmacological research. He has been consistently involved in drug discovery research and planning, in-licensing exploration, and development project management, and has been involved in many joint research and development projects with academia.
-
-

-
Deputy General Manager/Chief Strategist, Alliance Strategic Design Division, Rohto Pharmaceutical Co.Yasuo Sumida
President and Director of MG Pharma, Inc.
- After graduating from the Faculty of Pharmaceutical Sciences at Kyoto Pharmaceutical University and completing a master's degree at the same university, he joined Rohto Pharmaceutical in 1998 and was assigned to the Product Planning Department. He was involved in product planning in a wide range of areas including eye drops, oral medicines and supplements, functional cosmetics, etc. In 2006, he launched Research Village Kyoto (RVK) in the R&D Marketing Group, R&D Division, and from 2007, he was in charge of product planning for skin care cosmetics for 10 years, and in 2014, he was appointed PJ leader for HR reform as a W In 2018, he became Deputy General Manager of the Media & Promotion Department, and in 2020, he will assume his current position.
-
finalist
-
SOMPO Holdings Award

- Universal Bio Sampling, Inc.
- Representative] Fumiaki Hirata

Innovative high-throughput specimen testing system
Innovative high-throughput testing method that solidifies liquid specimens on a card with a unique QR code. Enables specimens to be transported and stored at room temperature for long periods of time, and provides manpower-saving services by realizing easy tracing and personal information protection management through ICT linkage. -
Sysmex Award

- HiLung Co.
- Representative] Shunsuke Ito
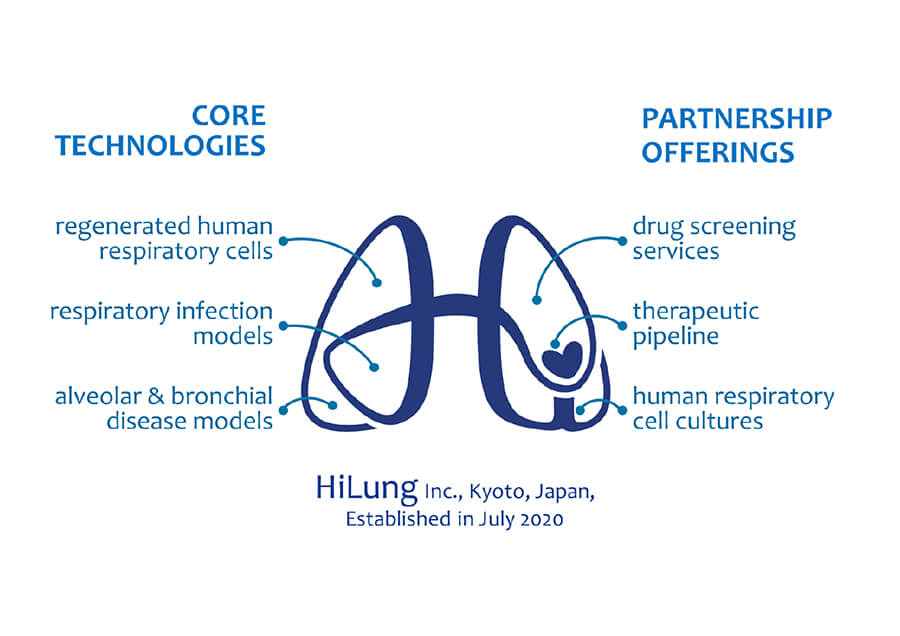
Stem Cell Technology Revolutionizes the Respiratory Drug Discovery Process
The lung is a critical organ exposed to external risks such as infection and pollution, but the success rate of drug development is low due to disease model inefficiencies, resulting in 10 million deaths/year worldwide. We aim to develop an innovative iPS drug discovery platform to fulfill the UMN requirement for this disease. -
Daiichi Sankyo Award

- Veneno Technologies, Inc.
- Representative] Hikaru Taira
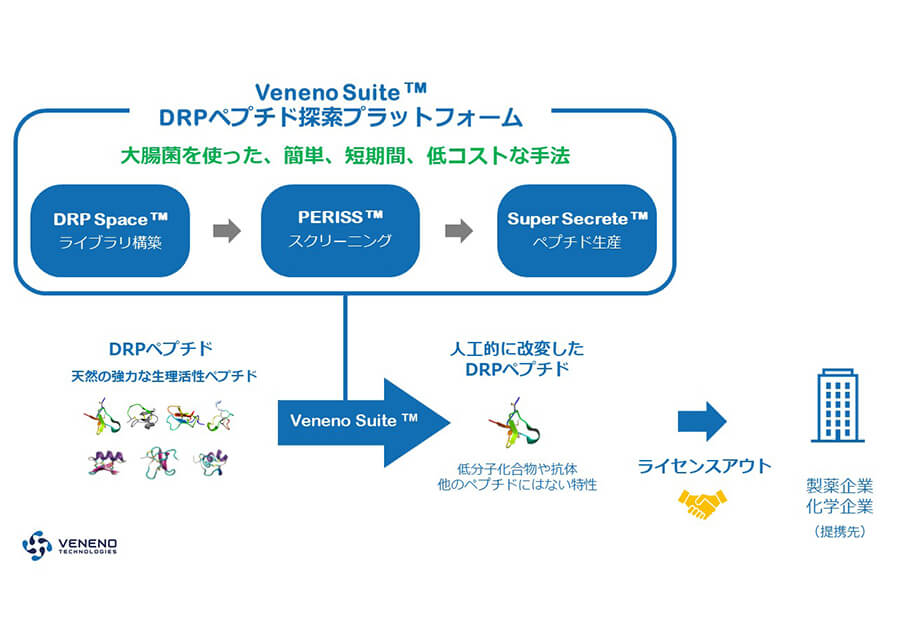
Peptide Drug Development by Innovative Screening Technology
Artificially evolve natural, highly pharmacologically active peptides using proprietary peptide screening technology based on evolutionary molecular engineering to develop innovative peptide drugs (injectable drugs with overwhelmingly long half-lives in blood, highly selective ion channel drugs, etc.). -

- Mill Ion Co.
- Representative] Kazuki Kotake
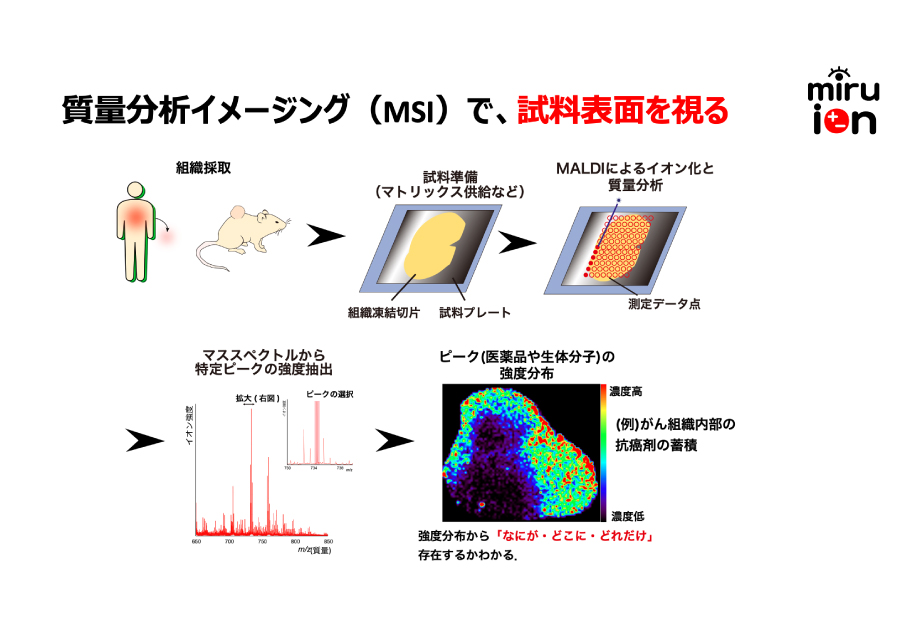
Visualize any health history through hair testing
By applying mass spectrometry imaging, which can visualize "what molecules are present, where they are present, and how many are present," to hair analysis, we have succeeded in showing changes in health status in a "visible" form. This will realize a society in which everyone can easily grasp the state of their health. -
Meiji Holdings Award

- Liquid Mine Co.
- Representative] Michikazu Kishimoto
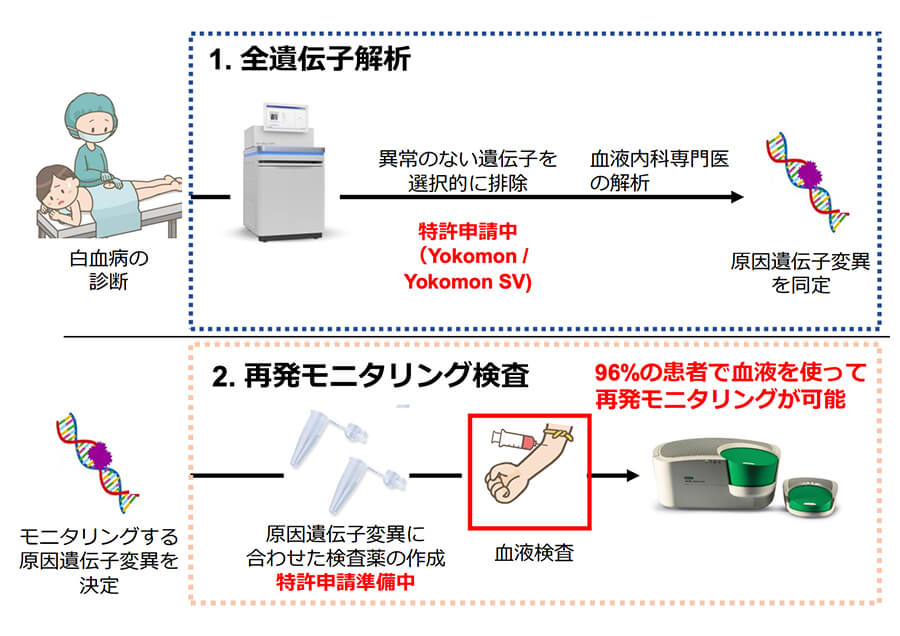
Monitoring test for early detection of leukemia relapse
Current leukemia relapse monitoring tests are available to only a few patients, and in many cases patients must undergo bone marrow testing. Through genetic analysis, we have established a technology that can be applied to patients with 96%, and monitoring is possible. -
Dai-ichi Life Holdings Award

- Almed Co.
- Representative] Tomoaki Shirao
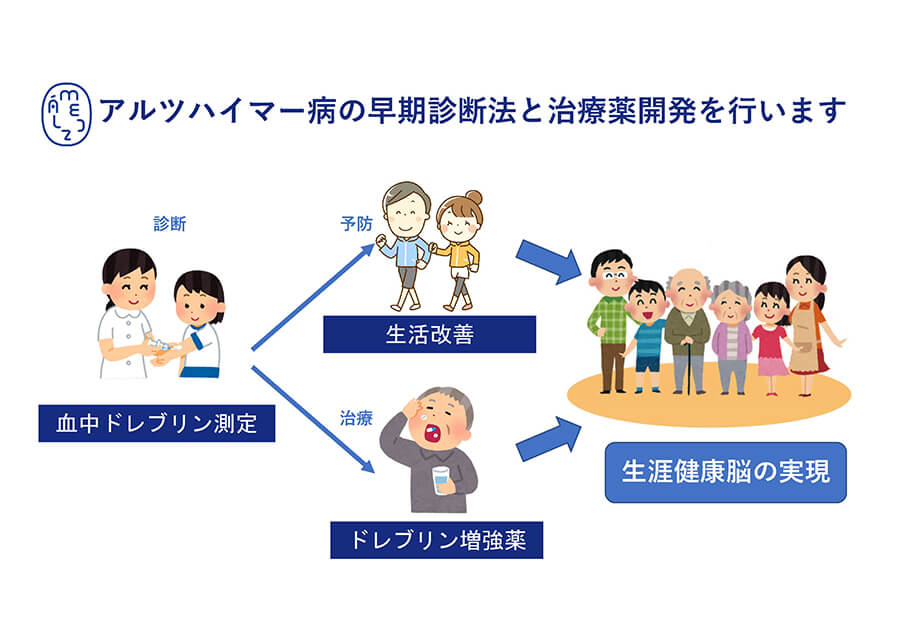
Creating a New Diagnostic and Therapeutic Agent for Dementia with Dreflin
There is no fundamental cure in Alzheimer's disease. We will develop the first ever diagnostic agent using the biomolecule drebrin and a therapeutic agent for Alzheimer's disease with a completely different mechanism than before, and realize a society in which the elderly with dementia can live in peace. -
Senju Pharmaceutical Award

- aceRNA Technologies, Inc.
- Representative] Fumihiro Sugawa
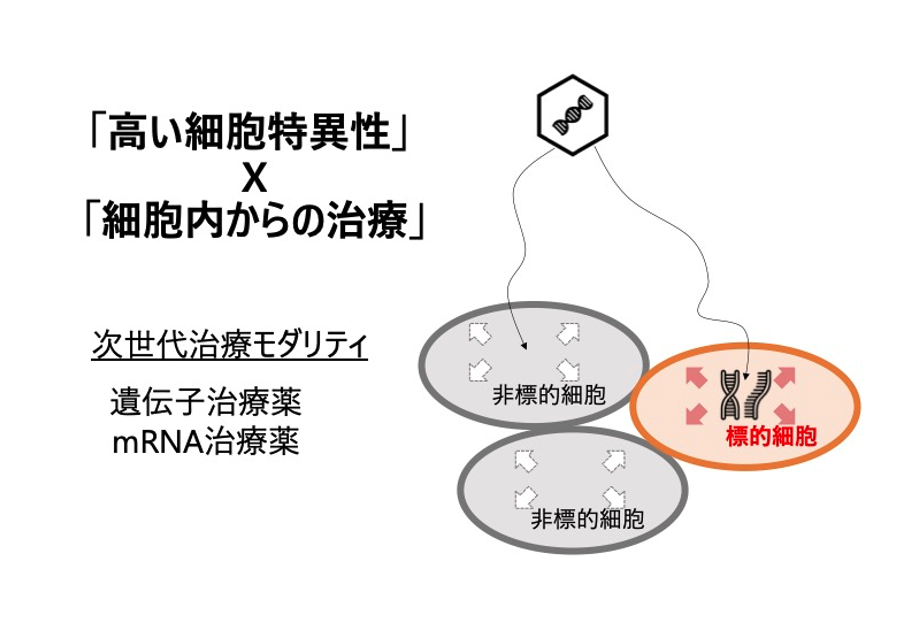
In vivo cell regulation and reprogramming by mRNA medicine
The mRNA drugs that are currently the focus of attention still face technical issues such as controlling expression sites and expression amounts, etc. Using RNA design technology developed through the accumulation of miRNA research, the company aims to realize "smart mRNA drugs" that can provide treatment according to the state of the cell. -
Kawarusaki Award

- Restoration Vision Inc.
- Representative] Yusaku Katada
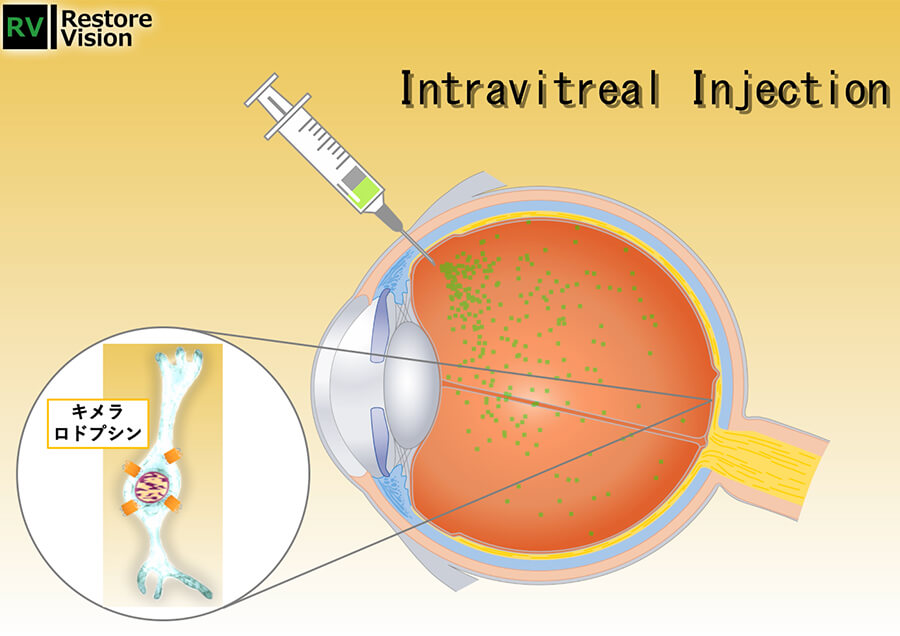
Development of a vision-regenerating gene therapy for retinitis pigmentosa
Retinitis pigmentosa is the second leading cause of visual impairment in Japan, and no effective treatment has been established. We will develop a chimeral rhodopsin-based visual regeneration gene therapy drug and provide it to patients around the world to eradicate blindness. -
Kobe Medical Industry Development Award

- Bio IT
- Representative] Masanori Ikeda
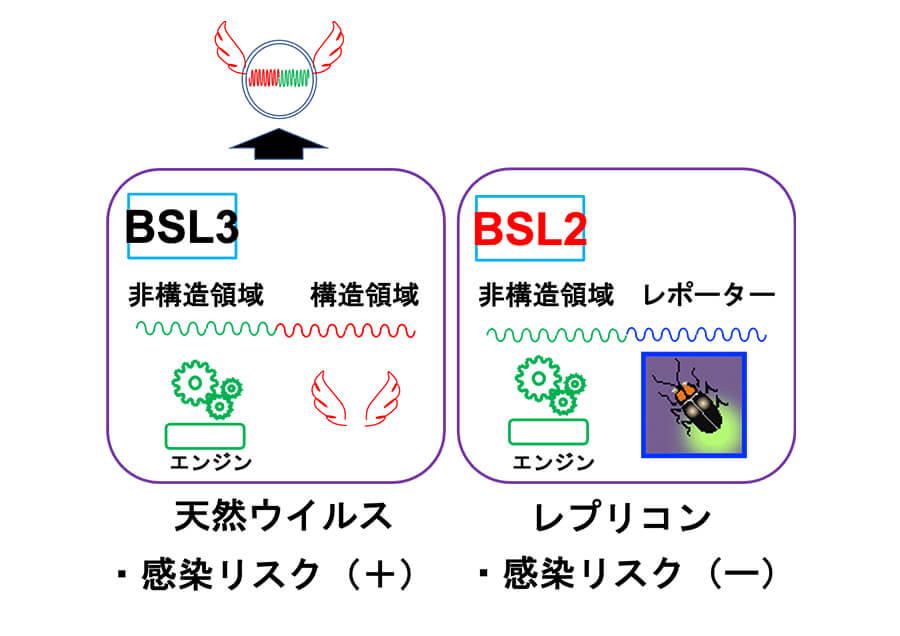
Protecting the World from Pandemics with Safe Man-Made Viruses
Developed a safe, non-infectious engineered virus and discovered a candidate antiviral agent against SARS-CoV-2, the cause of COVID-19. Establish a system that can protect the world from future pandemics caused by unknown viruses. -
Grand Prize / Taisho FRC Award

- RNart
- Representative] Yosuke Katsuta
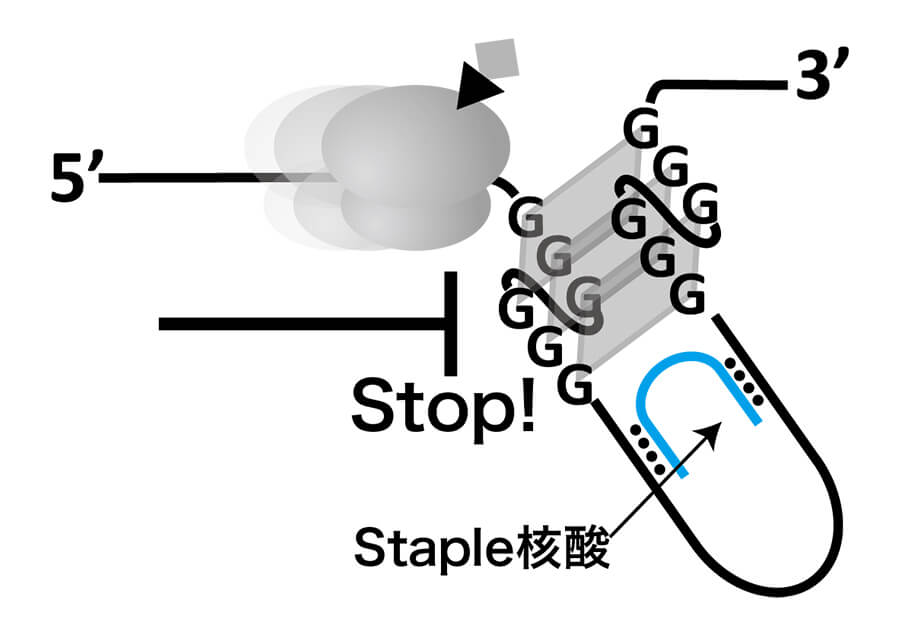
Gene Therapy with Staple Nucleic Acid
Staple nucleic acids have a high degree of freedom in design because they do not require linkage with enzymes, and they control gene expression by changing the structure of the target RNA. We propose the application of this technology in medicine, which eliminates the off-target effects that are a weak point of nucleic acid drugs. -
Real Tech Fund Award

- Team AMATERAS
- Representative] Taishi Kakizuka
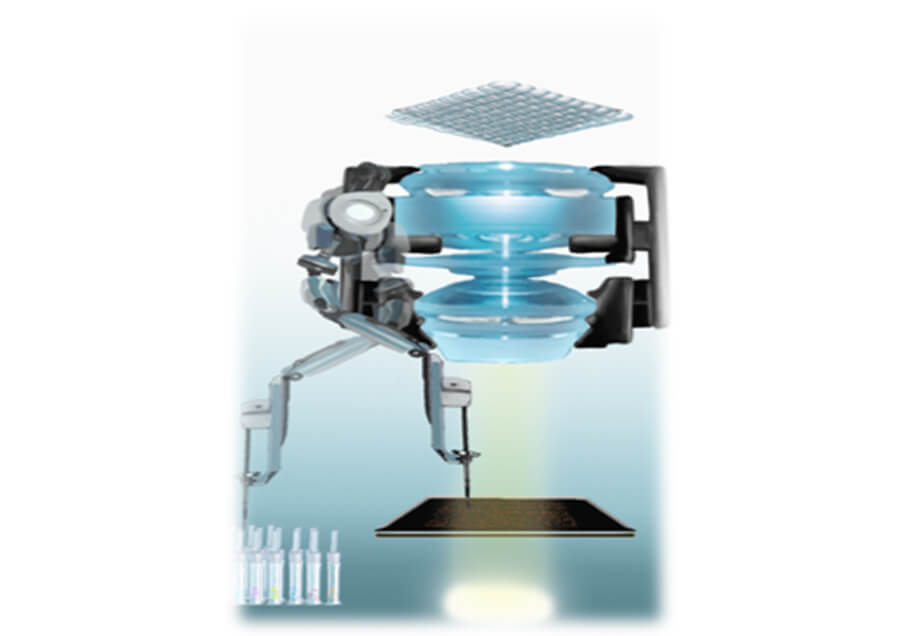
Realization of cell-level resolution and high-speed ultra-wide-field imaging
Developed AMATERAS, a high-speed ultra-wide-field imaging system that enables scanless, high-speed imaging at a cellular resolution of 1.5 cm scale. AMATERAS solves imaging problems in pathological diagnosis and regenerative medicine product testing, which have been limited to microscopic observation. -
Roth Prize

- liquid liver
- Representative] Nobuhiko Kojima

Liquid liver development
Phenylketonuria patients cannot metabolize phenylalanine in the liver and cannot eat meat for the rest of their lives. To treat diseases caused by these metabolic enzyme polymorphisms, we are developing a "liquid liver," a red blood cell that encapsulates metabolic enzymes and has a liver function.

